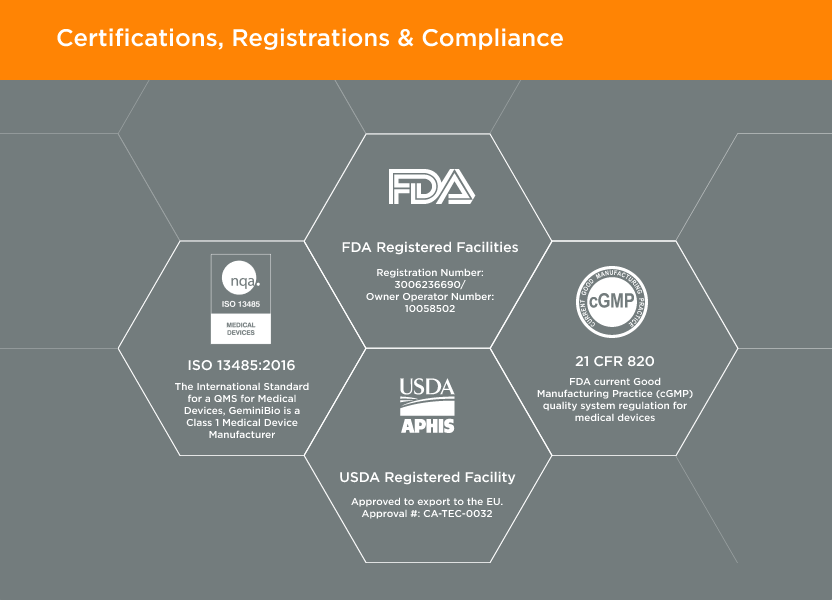
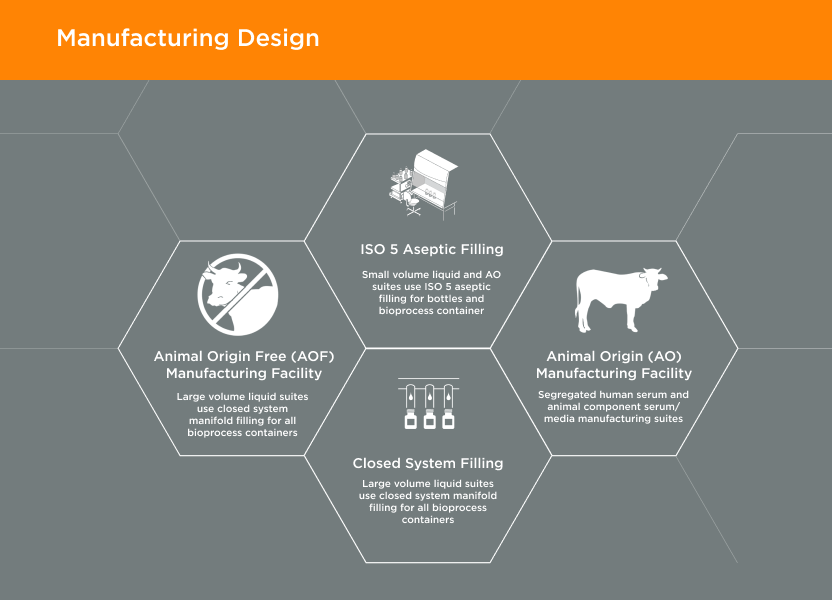
Doing the right thing – the first time. Honoring and keeping commitments.


Working with speed and focus to deliver the highest quality results – on time.


Embracing unique perspectives and treating others with dignity and respect.


Biased to improve processes and products to better serve customers and improve workflows.