Human Serum AB (HSAB)
Overview
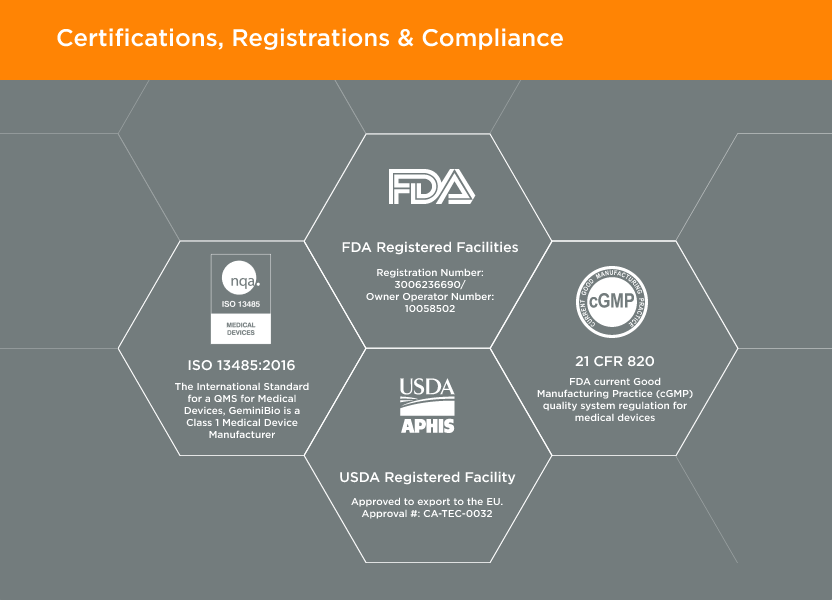
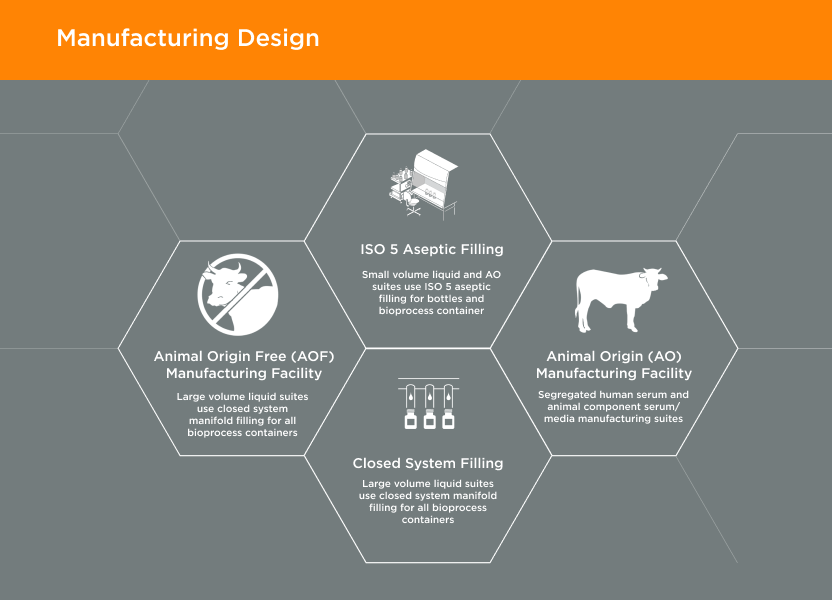
GeminiBio offers a wide range of HSAB quality options – manufactured under cGMP – with extensive testing, and the ability to transition to non-bovine component products to meet regulatory requirements, improving advanced therapy customer workflow productivity and ability to scale.