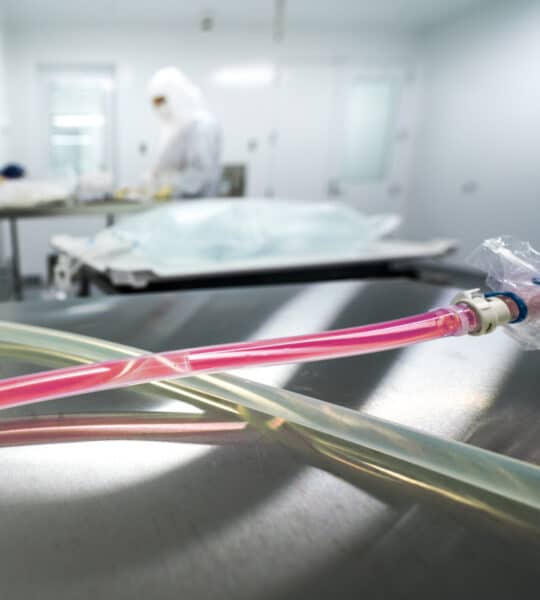
GeminiBio’s Aseptic Assurance System™ Supports Closed System Manufacturing for Cell Therapies
Gemini Bioproducts, LLC ("GeminiBio"), a leading supplier of cell culture reagents and process liquids, today announced the launch of new products designed to improve cell therapy manufacturing processes.
News
Mar 14, 2024