GeminiBio
What workflow solution are you looking for?

Overview
We enable our customers to optimize their workflow and productivity – from basic research to commercial production – to accelerate the development of life-enhancing biotherapeutics.
GeminiBio has two separate cGMP manufacturing facilities, comprising a total of 57,000 square feet, which are segregated between animal origin-free and animal component manufacturing.
Empowering Cell Culture and Process Liquid Workflows

About Us
Founded in 1985, GeminiBio serves the global biotechnology industry, from basic research to commercial production, with a focus on helping our customers accelerate the development of life-enhancing biotherapeutics by streamlining and improving their cell culture and process liquid manufacturing workflows.
GeminiBio’s products are organized into two core verticals – Cell Culture Solutions and Process Liquid Solutions.
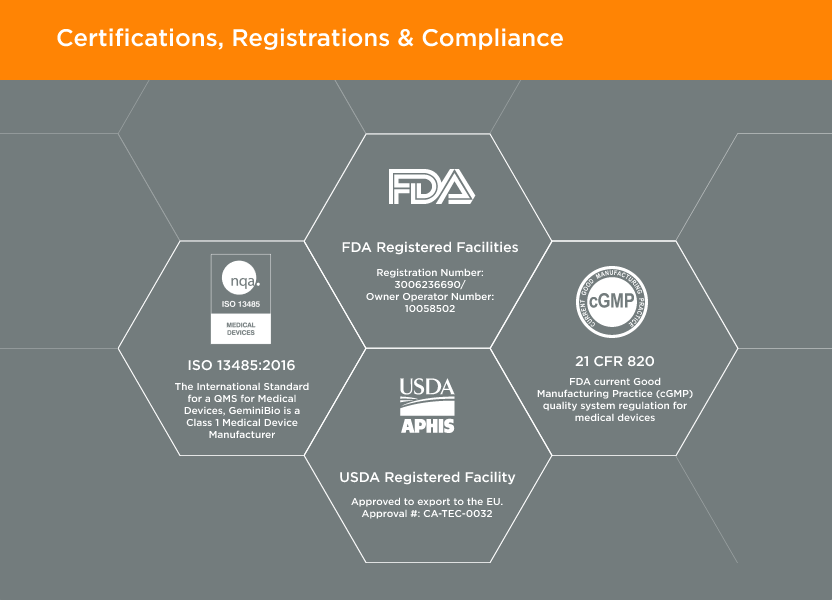
Located in West Sacramento, California; GeminiBio is an ISO 13485 certified, FDA registered Class 1 Medical Device Manufacturer, aligned with 21 CFR Part 820.
EST. 1985

Who We Serve
GeminiBio enables researchers to optimize their cell culture workflow to improve productivity, reproducibility, and speed. Our product portfolio includes a comprehensive line of fetal bovine serum (FBS), human serum (HSAB), other animal sera, cell culture media, supplements and reagents, growth factors and cytokines, plus lab consumables.

GeminiBio enables biopharma customers to optimize their upstream and downstream workflows to improve productivity and speed. Our product portfolio includes custom cGMP manufactured media, buffers, and other process liquids in batch sizes from 10-liters to 10,000-liters, and a comprehensive line of sera, including fetal bovine serum (FBS), as well as “off the shelf” water for injection (WFI) quality water.

GeminiBio enables gene therapy customers to optimize their upstream and downstream workflows to improve productivity and speed. We support the unique needs of plasmid DNA and viral vector manufacturing by providing custom manufactured cGMP media, buffers, and other process liquids in batch sizes from 10-liters to 10,000-liters, as well as “off the shelf” water for injection (WFI) quality water.

GeminiBio enables cell therapy customers to optimize their upstream and downstream workflows to improve productivity and speed. We support the unique needs of plasmid DNA and viral vector manufacturing, as well as therapeutic cell manufacturing, by providing custom manufactured cGMP media, buffers, and other process liquids in batch sizes from 10-liters to 10,000-liters. GeminiBio supports therapeutic cell manufacturing with a comprehensive line of human serum products, and mesenchymal stem cell (MSC) and T-Cell media.

GeminiBio enables life science tools manufacturers to significantly expand the scale – and flexibility – of their cGMP process liquid manufacturing, allowing them to better meet demanding end-customer requirements. Through our cGMP process liquid contract (private label) manufacturing capabilities, which include cGMP batch sizes from 10-liters to 10,000-liters, and ability to fill into diverse container types, and sizes ranging from 500 mL bottles to 1,000-liter pallet tanks, life science tools manufacturers can optimize their market penetration and end-customer satisfaction.

GeminiBio enables researchers to optimize their cell culture workflow to improve productivity, reproducibility, and speed. Our product portfolio includes a comprehensive line of fetal bovine serum (FBS), human serum (HSAB), other animal sera, cell culture media, supplements and reagents, growth factors and cytokines, plus lab consumables.

GeminiBio enables biopharma customers to optimize their upstream and downstream workflows to improve productivity and speed. Our product portfolio includes custom cGMP manufactured media, buffers, and other process liquids in batch sizes from 10-liters to 10,000-liters, and a comprehensive line of sera, including fetal bovine serum (FBS), as well as “off the shelf” water for injection (WFI) quality water.

GeminiBio enables gene therapy customers to optimize their upstream and downstream workflows to improve productivity and speed. We support the unique needs of plasmid DNA and viral vector manufacturing by providing custom manufactured cGMP media, buffers, and other process liquids in batch sizes from 10-liters to 10,000-liters, as well as “off the shelf” water for injection (WFI) quality water.

GeminiBio enables cell therapy customers to optimize their upstream and downstream workflows to improve productivity and speed. We support the unique needs of plasmid DNA and viral vector manufacturing, as well as therapeutic cell manufacturing, by providing custom manufactured cGMP media, buffers, and other process liquids in batch sizes from 10-liters to 10,000-liters. GeminiBio supports therapeutic cell manufacturing with a comprehensive line of human serum products, and mesenchymal stem cell (MSC) and T-Cell media.

GeminiBio enables life science tools manufacturers to significantly expand the scale – and flexibility – of their cGMP process liquid manufacturing, allowing them to better meet demanding end-customer requirements. Through our cGMP process liquid contract (private label) manufacturing capabilities, which include cGMP batch sizes from 10-liters to 10,000-liters, and ability to fill into diverse container types, and sizes ranging from 500 mL bottles to 1,000-liter pallet tanks, life science tools manufacturers can optimize their market penetration and end-customer satisfaction.
Our Integrated Quality Approach
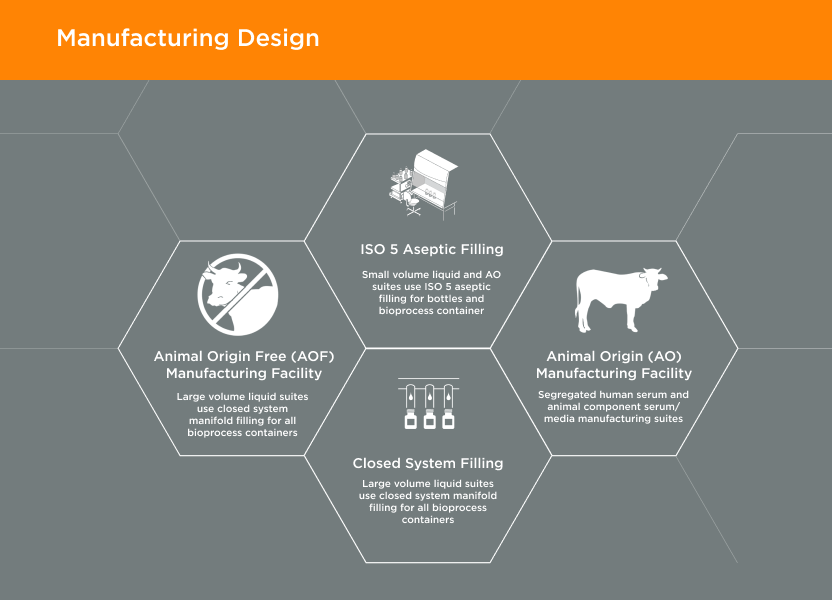
Manufacturing Design02
GeminiBio’s facilities are designed with current Good Manufacturing Practices (cGMP) and our customer’s quality requirements in mind. Our animal origin free (AOF) manufacturing facility is 1/4 mile away from our animal origin (AO) manufacturing facility. In addition, our cGMP warehouse is designed with flows for both personnel and materials, including segregation of AO and AOF materials.

How Can We Help?
Select from drop down
Contact: United States Sales Team
920 Stillwater Rd Suite 130,
West Sacramento, CA 95605,
United States of America
920 Stillwater Rd Suite 130,
West Sacramento, CA 95605,
United States of America
920 Stillwater Rd Suite 130,
West Sacramento, CA 95605,
United States of America
920 Stillwater Rd Suite 130,
West Sacramento, CA 95605,
United States of America
920 Stillwater Rd Suite 130,
West Sacramento, CA 95605,
United States of America
920 Stillwater Rd Suite 130,
West Sacramento, CA 95605,
United States of America